
Rather, the aim is to illustrate some concepts that facilitate the development of scientifically justified sampling plans that need not represent an unnecessary burden on industry.įrequently, it is perceived that an increased sample size is necessary for larger batch sizes. It is not the intention of this paper to prescribe an approach that represents the best fi t for all biopharmaceutical companies or product presentations.
Where the integrity of each individual container is assured by a suitably qualified process, additional CCI sampling and testing would not normally be required. These approaches prevent unnecessary sampling and testing while retaining high product quality. This paper summarizes a practical and risk-based strategy, both representing scientifically justified approaches. This is a particularly important consideration when using destructive test methods. This paper provides some considerations and guidance on defining a justifiable sample size for CCIT when required for parenteral biopharmaceutical finished products.

However, it is accepted and appropriate that, when sampling is required, scientifically valid sampling plans should be based on risk assessment, including information such as supplier approval, packaging component specifications and process knowledge

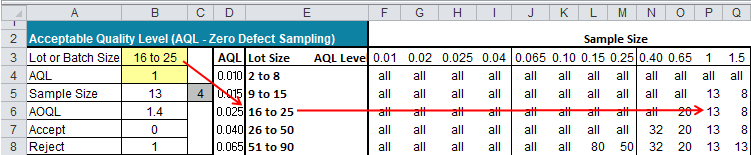
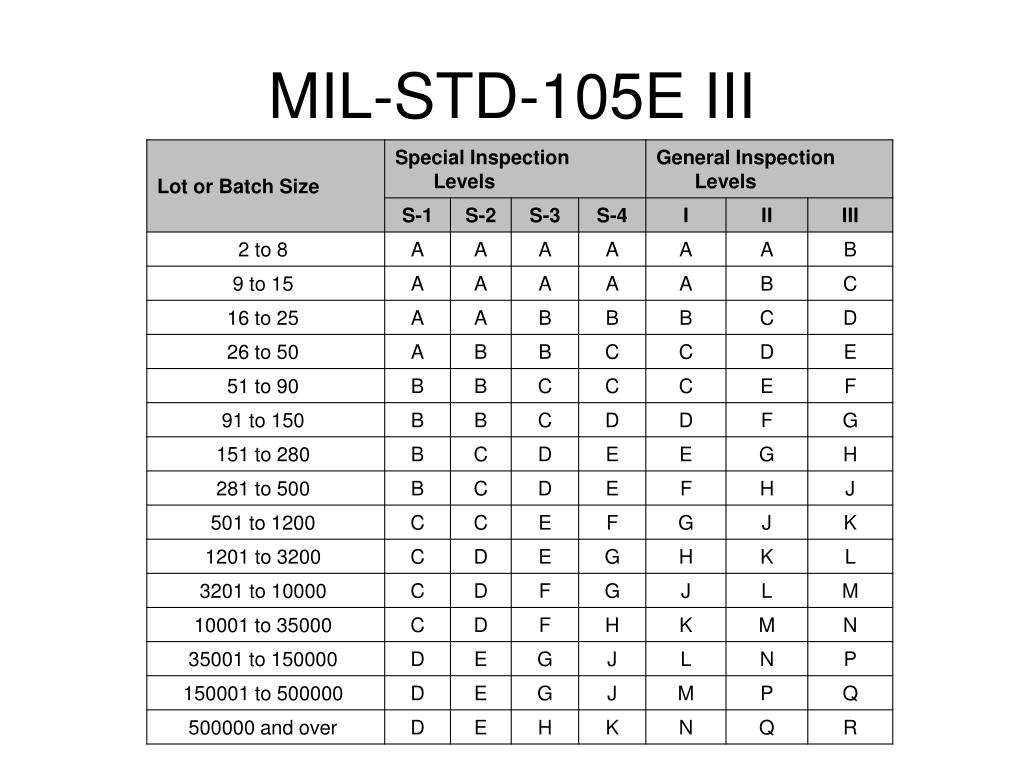
This industry group considers that a holistic approach 3,4 is preferable to sampling and testing for assuring CCI. CCIT in support of container closure system (CCS) development, process validation activities or stability testing are also out of scope. This is consistent with current regulatory guidance, which does not require CCIT for every container, or for every batch, unless the container is sealed by fusion, 2 which is out of the scope of this paper. 1 CCIT is not a batch release requirement for the majority of companies some companies may sample lots for CCIT as part of in-process control (IPC) or as a characterization test to monitor performance of the entire manufacturing process. Unpublished industry surveys highlight that the majority of leading biopharmaceutical companies do not routinely perform CCIT for each batch of a commercial product but rather rely on a holistic approach guaranteeing consistent quality of the finished parenteral product with respect to CCI (container closure integrity). Currently, there is no official standard or published procedure to follow for sampling approaches or defining appropriate sample sizes. Sample size approaches for container closure integrity testing (CCIT) for routine commercial biopharmaceutical production vary across the industry.


 0 kommentar(er)
0 kommentar(er)
